Isotope and ion practice worksheet – Delve into the fascinating world of isotopes and ions with our comprehensive practice worksheet. This resource provides a thorough understanding of these fundamental concepts, their types, and their pivotal roles in chemical reactions.
Explore the practical applications of isotopes in diverse fields like medicine, industry, and research. Discover the significance of ions in electrochemistry, materials science, and environmental science. Engage with real-world examples that showcase the practical implications of these concepts.
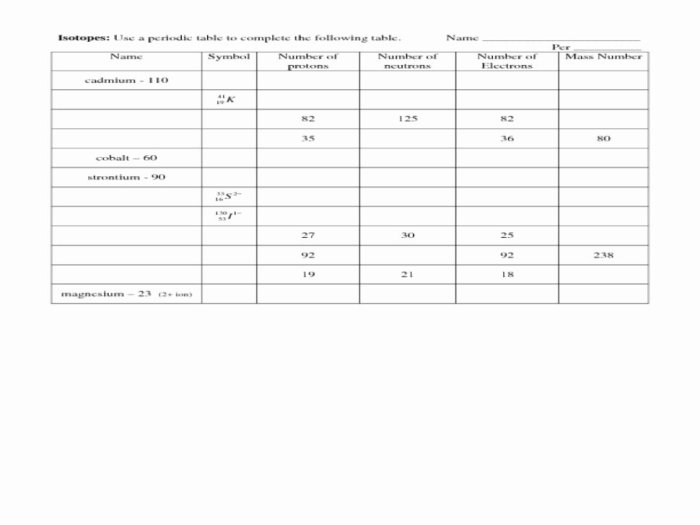
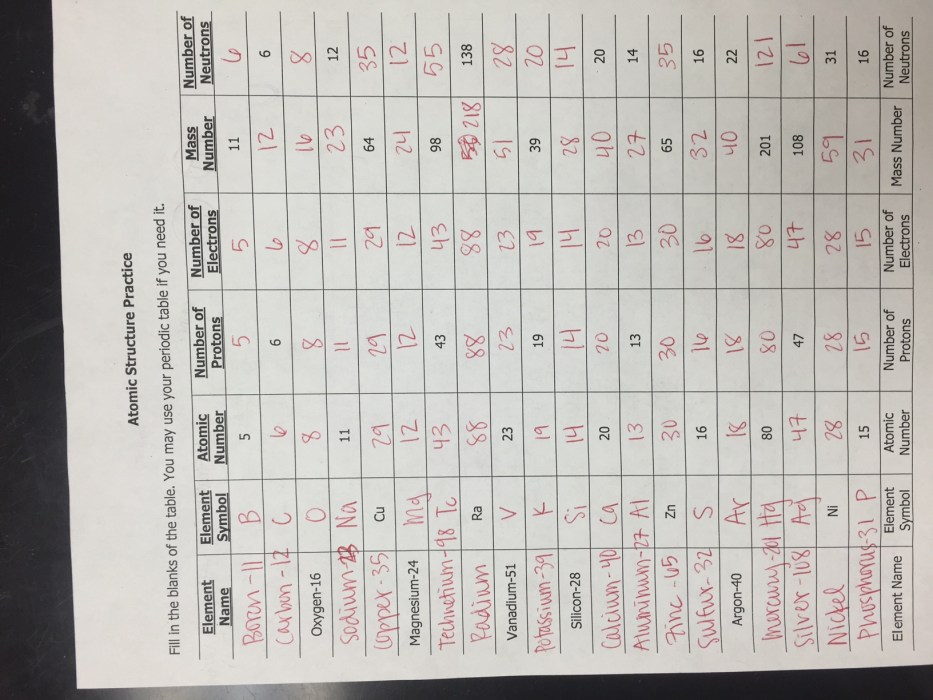
Isotopes: Isotope And Ion Practice Worksheet
Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. This means that isotopes have the same atomic number but different mass numbers.
There are three main types of isotopes: stable isotopes, radioactive isotopes, and unstable isotopes. Stable isotopes do not undergo radioactive decay, while radioactive isotopes do. Unstable isotopes are radioactive isotopes that have a short half-life, meaning that they decay quickly.
Examples of Isotopes and Their Uses
- Carbon-12is a stable isotope of carbon that is used in dating organic materials.
- Carbon-14is a radioactive isotope of carbon that is used in dating organic materials.
- Uranium-235is a radioactive isotope of uranium that is used in nuclear reactors.
Ions
Ions are atoms or molecules that have lost or gained electrons, resulting in a net electric charge. Ions can be either positive or negative.
There are two main types of ions: cations and anions. Cations are positively charged ions, while anions are negatively charged ions.
Examples of Ions and Their Uses
- Sodium ions (Na+)are cations that are essential for maintaining fluid balance in the body.
- Chloride ions (Cl-)are anions that are essential for maintaining fluid balance in the body.
- Hydrogen ions (H+)are cations that are essential for many chemical reactions.
Isotopes and Ions in Chemical Reactions
Isotopes and ions can play a significant role in chemical reactions. Isotopes can affect the rate of a reaction, while ions can affect the products of a reaction.
For example, the rate of a reaction can be increased by using an isotope of the reactant that has a higher mass number. This is because the heavier isotope will have a lower energy and will therefore be more likely to react.
Ions can also affect the products of a reaction. For example, the products of a reaction can be changed by using an ion of the reactant that has a different charge.
Examples of Chemical Reactions that Involve Isotopes and Ions
- The reaction of hydrogen gas with oxygen gas to form wateris a reaction that involves isotopes. The reaction can be sped up by using an isotope of hydrogen that has a higher mass number, such as deuterium.
- The reaction of sodium metal with chlorine gas to form sodium chlorideis a reaction that involves ions. The reaction can be changed by using an ion of sodium that has a different charge, such as sodium ion.
Applications of Isotopes and Ions
Isotopes and ions have a wide range of applications in medicine, industry, and research.
Applications of Isotopes, Isotope and ion practice worksheet
- Medicine:Isotopes are used in a variety of medical applications, such as cancer treatment, medical imaging, and dating fossils.
- Industry:Isotopes are used in a variety of industrial applications, such as food irradiation, oil exploration, and nuclear power.
- Research:Isotopes are used in a variety of research applications, such as dating archaeological artifacts, studying climate change, and tracing the movement of animals.
Applications of Ions
- Electrochemistry:Ions are used in a variety of electrochemical applications, such as batteries, fuel cells, and electroplating.
- Materials science:Ions are used in a variety of materials science applications, such as semiconductors, superconductors, and nanomaterials.
- Environmental science:Ions are used in a variety of environmental science applications, such as water treatment, air pollution control, and soil remediation.
FAQ Compilation
What are isotopes?
Isotopes are atoms of the same element with the same number of protons but different numbers of neutrons.
What are ions?
Ions are atoms or molecules that have lost or gained electrons, resulting in a net electric charge.
How do isotopes and ions affect chemical reactions?
Isotopes and ions can affect the rate and outcome of chemical reactions by altering the mass and charge of the reactants and products.